Andrei Alyokhin, Mitchell Baker, David Mota-Sanchez, Galen Dively, and Edward Grafius
This peer-refereed invited review article has been first published in the December, 2008, issue of the American Journal of Potato Research (Volume 85, Number 6, Pages 395-413). The original publication is available at www.springerlink.com. Reproduced with permission of the publisher.
ABSTRACT
The Colorado potato beetle, Leptinotarsa decemlineata (Say), is widely regarded as the most important insect defoliator of potatoes. Its current range covers about 16 million km2 in North America, Europe, and Asia and continues to expand. This insect has a complicated and diverse life history, which is well-suited to agricultural environments, and makes it a complex and challenging pest to control. Dispersal, closely connected with diapause, feeding, and reproduction, allow the Colorado potato beetle to employ "bet-hedging" reproductive strategies, distributing its offspring in both space (within and between fields) and time (within and between years).
The Colorado potato beetle played a large role in creating the modern pesticide industry, with hundreds of chemicals tested against it. High selection pressure, together with natural propensity to adapt to toxic substances, eventually resulted in a large number of insecticide-resistant Colorado potato beetle populations. Since the middle of the last century, the beetle has developed resistance to 52 different compounds belonging to all major insecticide classes. Resistance levels vary greatly among different populations and between beetle life stages, but in some cases can be very high (up to 2,000-fold).
Known mechanisms of Colorado potato beetle resistance to insecticides include enhanced metabolism involving esterases, carboxylesterases and monooxygenases, and target site insensitivity, as well as reduced insecticide penetration and increased excretion. There is also some evidence of behavioral resistance. Resistance mechanisms are sometimes highly diverse even within a relatively narrow geographical area. Resistance is usually inherited as an incompletely dominant or incompletely recessive trait, with one or several genes involved in its determination. Because of pleiotropic effects of resistant alleles, insecticide-resistant beetles often have reduced relative fitness in the absence of insecticides.
Rotating different classes of insecticides and reducing insecticidal pressure on pest populations by provision of temporal and spatial refuges from exposure to toxins have been proposed to delay evolution of resistance. However, insecticide resistance in this insect will likely remain a major challenge to the pest control practitioners. Still limited understanding of beetle biology, its flexible life history, and grower reluctance to adopt some of the resistance management techniques create impediments to successful resistance management. Overcoming these obstacles is not an easy task, but it will be crucial for sustainable potato production.
COLORADO POTATO BEETLE AS AN INTERNATIONAL SUPERPEST
Colorado potato beetle, Leptinotarsa decemlineata (Say), is a leaf beetle (Coleoptera: Chrysomelidae) native to the southwestern United States and Mexico. It was first collected in 1811 by Thomas Nuttall and then described by Thomas Say in 1824 (Jacques 1988). Wild populations were observed feeding on buffalobur, Solanum rostratum Dunal. and several related species in the family Solanacea. For more than four decades after its original discovery, the beetle was of little practical significance. However, the situation changed dramatically after the area of its original distribution was settled by farmers of European descent. The settlers brought with them potato, Solanum tuberosum L., and started to cultivate it over considerable acreages. Unfortunately, the potato proved to be a highly suitable host plant for L. decemlineata, and the beetles quickly switched to feeding on the new and abundant host plant (Casagrande 1987; Jacques 1988; Weber 2003).
The first major Colorado potato beetle outbreak occurred in 1859 on potato fields about 100 miles west of Omaha, Nebraska (Jacques 1988). The subsequent expansion in beetle geographic range was somewhat mind-boggling, with beetles reaching the Atlantic coast of the U.S. and Canada before 1880 (Casagrande 1987). The first European population was established in France in 1922. By the end of the 20th century, the pest had become a problem all over Europe, in Asia Minor, Iran, Central Asia, and western China (Jolivet 1991; Weber 2003). Its current range covers about 16 million km2 on two continents and continues to expand (Weber 2003). Potentially, the beetle could spread to temperate areas of East Asia, the Indian subcontinent, South America, Africa, New Zealand, and Australia (Vlasova 1978; Worner 1988; Jolivet 1991; Weber 2003).
Currently, the Colorado potato beetle is widely regarded as the most important insect defoliator of potatoes. It also causes significant damage to tomato and eggplant. One beetle consumes approximately 40 cm2 of potato leaves during the larval stage (Logan et al. 1985; Ferro et al. 1985), and close to an additional 10 cm2 of foliage per day as an adult (Ferro et al. 1985). In addition to impressive feeding rates, the Colorado potato beetle is also characterized by high fecundity, with one female laying 300-800 eggs (Harcourt 1971). If left uncontrolled, the beetles can completely destroy potato crops.
LIFE HISTORY
The Colorado potato beetle has a complicated and diverse life history, which is well-suited to agricultural environments, and makes it a complex and challenging pest to control. Dispersal, closely connected with diapause, feeding, and reproduction, allow this insect to employ "bet-hedging" reproductive strategies, distributing its offspring in both space (within and between fields) and time (within and between years). As a result, the risk of catastrophic losses of offspring due to insecticides or crop rotation is diminished (Solbreck 1978; Voss and Ferro 1990).
The Colorado potato beetle is capable of moving both by flight and by walking (Boiteau et al. 2003). Adults start walking immediately after they emerge from pupation in the soil (Voss and Ferro 1990) and can travel several hundred meters. Walking speed is the highest on bare ground and decreases with the increasing density of vegetation (Ng and Lashomb 1983). Adult beetles need to feed for 5-10 days after eclosion from pupae to complete flight muscle development (Voss and Ferro 1990; Yang 1994; Weber and Ferro 1996), and then they can easily fly several kilometers (Weber and Ferro 1994). Flight initiation is strongly related to air temperature and solar insolation (Caprio and Grafius 1990), and given favorable wind direction the beetles may be able to fly over 100 km (Wiktelius 1981). The beetles also fly over short distances, distributing eggs within the host habitat and in search of mates (Voss and Ferro 1990).
The Colorado potato beetle overwinters in the soil as adults. After diapause is induced by a short-day photoperiod (de Kort 1990), the beetles may enter the soil for diapause in the potato field or move towards field edges by flight and by walking, presumably orienting towards tall vegetation common in hedgerows (Voss and Ferro 1990; Weber and Ferro 1993; French et al. 1993). Upon arrival to overwintering sites, pre-diapause migrants immediately burrow into the soil to diapause (Voss and Ferro 1990), and their flight muscles undergo significant degeneration (Stegwee et al. 1963). Although in some areas the majority of beetles aggregate in overgrown areas adjacent to fields where they have spent the previous summer (Weber and Ferro 1993; French et al. 1993), a substantial number burrow into the soil directly in the field (French et al. 1993).
Refractory phase of the diapause, during which the beetles do not react to the change in environmental conditions, lasts for approximately 3 months. After that, the beetles respond to elevation of temperatures above 10°C by emerging from the soil (de Kort 1990). The beetles usually accumulate 50-250 degree-days (DD10, 10°C base temperature) before they appear on the soil surface (Yang 1994; Ferro et al. 1999). Males and females terminate their diapause simultaneously (Yang 1994; Ferro et al. 1999), and require only 60-80 DD10 before they are able to mate (Ferro et al. 1999). A certain proportion of overwintering beetles may remain in the state of uninterrupted extended diapause for two years (Isely 1935; Trouvelot 1936; Wegorek 1957 a,b; Ushatinskaya 1962, 1966). In the western Ukraine, 0.4-6.5% of beetles overwintering in sandy soils remained dormant for two years, but all beetles overwintering in clay soils emerged after the first winter (Ushatinskaya 1962, 1966). Similarly, between 0-7.2% of beetles in upstate New York emerged after more than one winter in dormancy (Tauber and Tauber 2002). Incidence of prolonged diapause was even higher in Washington State, where 16-21% of overwintering adults emerged after two winters, and up to 2% emerged after three winters (Biever and Chauvin 1990).
After emergence from the soil, overwintered Colorado potato beetles colonize potato fields both by flight and by walking (Voss and Ferro 1990). The beetles do not start flying until they accumulate 150-200 DD10 (amount required for flight muscle regeneration, Yang 1994), and beetle flight is strongly encouraged by the absence of food (Caprio and Grafius 1990; Ferro et al. 1991; Weber and Ferro 1996; Ferro et al. 1999; but see MacQuarrie and Boiteau 2003). If the fields are rotated, the beetles are able to fly up to several kilometers to find a new host habitat (Ferro et al. 1991; Weber and Ferro 1996). However, this is not a directed flight toward new fields and numbers of beetles arriving in new fields are greatly reduced and arrival is delayed (Weisz et al. 1996). Once they have colonized the field, the overwintered beetles first feed and then oviposit within 5-6 days (Ferro et al. 1985; Ferro et al. 1991).
Depending on temperature, development from egg to adult takes between 14-56 days (de Wilde 1948; Walgenbach and Wyman 1984; Logan et al. 1985; Ferro et al. 1985). The fastest development occurs between 25-32ºC and appears to differ among populations of different geographic origins. The larvae are capable of behavioral thermoregulation via moving within plant canopies (May 1981; Lactin and Holliday 1994), thus optimizing their body temperature compared to the ambient temperature. Diapause is facultative, and the beetles can have between one and three overlapping generations per year. The majority of adults die during the second summer of their lives. However, a certain proportion of overwintered beetles enter the second diapause in the following winter (Isely 1935; de Wilde 1962; Jermy and Saringer 1955; Minder and Petrova 1966). As many as 25% of the overwintered population may enter the second diapause (Minder and Petrova 1966), but overwintering and spring mortality among such beetles is very high (Isely 1935; Minder and Petrova 1966).
The beetle’s mating behavior is strongly directed towards maximizing genetic variability of its progeny. After accumulating at least 34DD10 since emergence from pupae (Alyokhin and Ferro 1999a), both males and females perform multiple copulations with different partners (Szentesi 1985). When a summer generation female mates to two different males, sperm precedence is incomplete, with the first male fertilizing 28-48% of the eggs (Boiteau 1988a; Alyokhin and Ferro 1999b; Roderick et al. 2003). Females do not usually start ovipositing until they accumulate at least 51 DD10 since emergence from pupae (Alyokhin and Ferro 1999a). Post-diapause females can lay eggs using sperm from the pre-diapause mating the previous fall; however, the number of offspring produced by such females is lower compared to spring-mated females (Ferro et al. 1991; Baker et al. 2005). Therefore, beetles usually mate again after diapause termination in the spring. Mating starts before beetles leave for the host habitat, with at least half of the population mating within the overwintering sites (Ferro et al. 1999). Sperm from spring mating typically takes precedence over overwintered sperm from the previous year's mating (Baker et al. 2005).
Mating status affects beetle flight activity. Unlike females from a number of other insect species (Johnson 1969; Dingle 1985), gravid Colorado potato beetle females continue to display a considerable amount of flight activity (Ferro et al. 1999; Alyokhin and Ferro 1999a), allowing them to distribute eggs within and between fields. Nevertheless, they still fly significantly less than unmated females (Alyokhin and Ferro 1999a), probably because both migration and reproduction are physiologically demanding for females, and these two processes are known to interfere with each other (Rankin et al. 1986). Contrary to mated females, mated males increase their flight activity, increasing the number of copulations with different mates (Alyokhin and Ferro 1999a) and genetic diversity of their offspring (McCauley and Reilly 1984). Unlike flight of the summer-generation beetles, flight of post-diapause beetles is not affected by their mating status (Ferro et al. 1999).
CHEMICAL CONTROL AND INSECTICIDE RESISTANCE
The Colorado potato beetle has been credited with being largely responsible for creating the modern insecticide industry (Gauthier et al. 1981). Since 1864, hundreds of compounds were tested against this pest, and application equipment was specifically invented to aid their delivery (Gauthier et al. 1981). The early chemicals of choice were arsenicals, such as Paris green and lead arsenate, and, to a smaller degree, botanical rotenone (Brown 1951). The beetles were also one of the initial targets for DDT applications, with initial applications as early as 1939 (Hitchner 1952; Gauthier et al. 1981). Currently, insecticides still remain the foundation of the Colorado potato beetle control on commercial potato farms. More than 30 active ingredients are currently registered for use against this pest in the United States (Table 1).
|
Group |
Subgroup |
Mode of Action |
Chemical group |
Trade names |
|
1 |
A |
Acetylcholine esterase inhibitors |
Carbamates |
Carbaryl, carbofuran, methomyl, oxamyl |
|
|
B |
|
Organophosphates |
Azinphos-methyl, dimethoate, disulfoton, ethoprop, malathion, methamidophos, parathion-methyl, phorate, phosmet |
|
2 |
A |
GABA-gated chloride channel antagonists |
Cyclodiene organochlorines |
Endosulfan |
|
3 |
|
Sodium channel modulators |
Pyrethroids, Pyrethrins |
Cyfluthrin, deltamethrin, esfenvalerate, permethrin |
|
4 |
A |
Nicotinic Acetylcholine receptor agonists / antagonists |
Neonicotinoids |
Acetamiprid, dinotefuran, imidacloprid, thiamethoxam, |
|
5 |
|
Nicotinic Acetylcholine receptor agonists (other than group 4) |
Spinosyns |
Spinosad |
|
6 |
|
Chloride channel activators |
Avermectins |
Abamectin |
|
9 |
|
Compounds of unknown or nonspecific mode of action (selective feeding blockers) |
Cryolite |
Cryolite |
|
11 |
|
Microbial disruptors of insect midgut membranes |
Bacillus thuringiensis subsp. tenebrionis endotoxins |
Bt |
|
15 |
|
Inhibitors of chitin biosynthesis, type 0, Lepidopteran |
Benzoylurea |
Novaluron |
|
18 |
B |
Ecdysone agonists / moulting disruptors |
Azadirachtin |
Neem |
|
22 |
|
Voltage-dependent sodium channel blocker |
Indoxacarb |
Indoxacarb |
|
25 |
|
Neuronal inhibitors |
Bifenazate |
Bifenazate |
Not surprisingly, high selection pressure eventually resulted in a large number of insecticide-resistant Colorado potato beetle populations, with resistance rapidly progressing since the middle of the last century (Fig. 1).
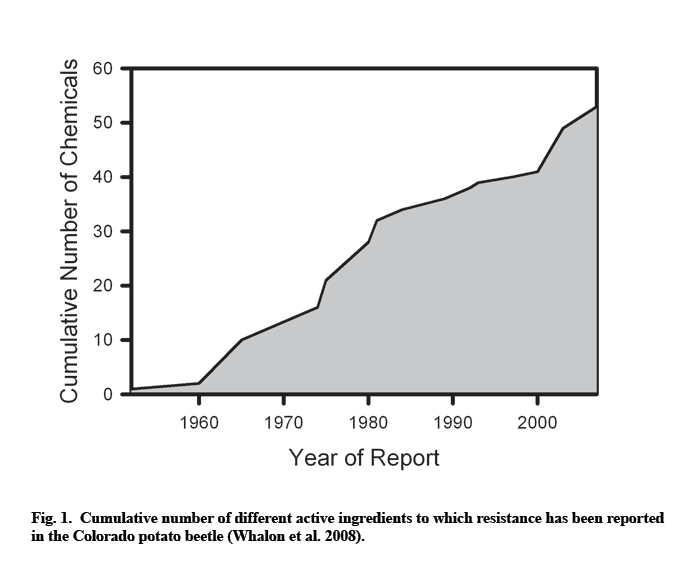
High predisposition to resistance development is probably caused by a number of factors. First, plants in the family Solanaceae have high concentrations of toxic glycoalkaloids in their foliage. Therefore, coevolution of the beetle and its host plants required development of the physiological capability to detoxify or tolerate poisons (Ferro 1993; Bishop and Grafius 1996). Secondly, high beetle fecundity both increases the probability of random mutations, as well as ensures the rapid build-up in numbers of resistant mutants once such mutations have occurred (Bishop and Grafius 1996). Thirdly, the beetles have a narrow host range, and both larvae and adults feed on the same host plants. This reduces the size of an unstructured refuge where susceptible genotypes may escape exposure to chemicals (Bishop and Grafius 1996; Whalon and Ferro 1998). Although some susceptible beetles may survive on untreated volunteer potatoes within fields rotated to alternate crops or on solanaceous weeds, their number is apparently insufficient for reducing the frequency of resistant alleles below the economically significant level. Fourthly, with the exception of crop rotation, growers rely almost exclusively on insecticides for beetle control because other control techniques do not provide a feasible alternative (Harcourt 1971; Casagrande 1987; Bishop and Grafius 1996). This increases selection pressure towards resistance. Finally, being native to North America, the Colorado potato beetle did not undergo genetic bottleneck typical of introduced pests (Hawthorne 2001; Weber 2003). Such genetic variability may provide evolutionary plasticity necessary for adaptation to adverse conditions. However, the relative importance of the last factor is unclear because resistance is also a problem in Europe, where the Colorado potato beetle is an introduced species (Weber 2003).
The first instance of Colorado potato beetle resistance to synthetic organic pesticides was noted for DDT in 1952 (Quinton 1955). Resistance to dieldrin was reported in 1958, followed by resistance to other chlorinated hydrocarbons (Hofmaster et al. 1967). In subsequent years, failures have been reported for most major classes of insecticides (Table 2). Although the northeastern United States is one of the main problem areas (Forgash 1985;
|
Chemical group |
Trade names |
|
Carbamates |
Aldicarb, carbaryl, carbofuran, cloethocarb, dioxacarb, oxamyl, propoxur |
|
Organophosphates |
Azamethiphos, azinphosethyl, azinphosmethyl, chlorfenvinphos, malathion, methamidophos, methidathion, monocrotophos, parathion, parathion-methyl, phorate, phosmet, phoxim, quinalphos, tetrachlorvinphos, trichlorfon |
|
Organochlorines |
DDT, methoxychlor |
|
Cyclodiene Organochlorines |
Aldrin, chlordane, dieldrin, endosulfan, endrin, HCH-gamma, toxaphene |
|
Organotins |
Azocyclotin |
|
Inorganics |
Hydrogen cyanide |
|
Pyrethroids, Pyrethrins |
Cypermethrin, deltamethrin, esfenvalerate, fenvalerate, permethrin |
|
Isoflavones |
Rotenone |
|
Neonicotinoids |
thiamethoxam, acetamiprid, clothianidin, dinotefuran, imidacloprid, N-desmethylthiamethoxam, N-methylimidaclopridnitenpyram, thiacloprid |
|
Macrocyclic lactones (avermectins) |
Abamectin |
|
Nereistoxin analogues |
Cartap |
|
Spinosyns |
Spinosad |
|
Bacillus thuringiensis subsp. tenebrionis endotoxins |
Bt |
Whalon and Ferro 1998), insecticide resistance in the Colorado potato beetle is a truly global phenomenon (Hofmaster et al. 1967; Forgash 1985; Boiteau 1988b; Ioannidis et al. 1991; Heim et al. 1992; Stewart et al. 1997; Noronha et al. 2001; Stankovic et al. 2004; Pourmirza 2005; Mota-Sanchez et al. 2006; Benkovskaya et al. 2006; Mohammadi Sharif et al. 2007; Whalon et al. 2008). Obviously, not every beetle population is resistant to every single compound that has ever been observed to fail against this pest. However, both cross-resistance and multiple resistance appear to be very common within the tested populations (Harris and Svec 1981; Ioannidis et al. 1991; Heim et al. 1992; Stewart et al. 1997; Mota-Sanchez et al. 2006; Alyokhin et al. 2006, 2007; Whalon et al. 2008).
Resistance problems reached critical levels in the U.S. in the early 1990s, when growers in some potato-producing regions completely ran out of chemical control options. Arrival of neonicotinoid insecticides in 1995 brought a period of relief in areas where the beetles had developed resistance to other chemicals (Whalon and Ferro 1998). However, the first instances of resistance to imidacloprid were soon reported from commercial potato farms in several U.S. states (Zhao et al. 2000; Olson et al. 2000; Mota-Sanchez et al. 2006; Alyokhin et al. 2006, 2007). While a number of new chemistries are expected to appear on the market in the near future, there is no reason to believe that any of them will break the seemingly endless insecticide – resistance – new insecticide cycle that is so characteristic of the Colorado potato beetle management (Fig. 2).
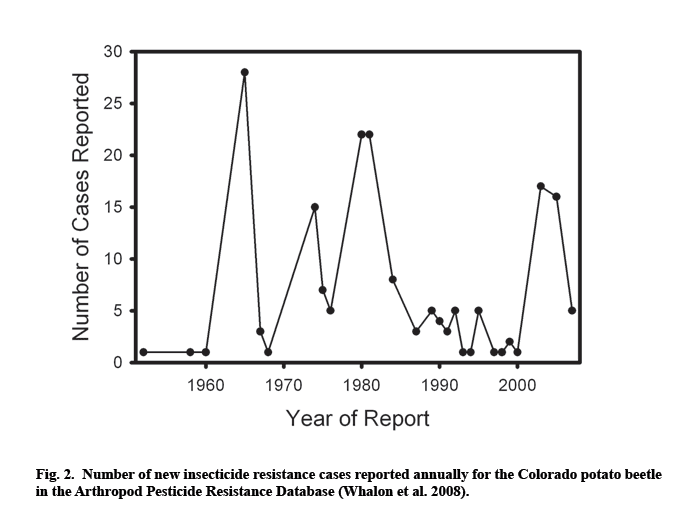
Insecticide resistance is likely to be a serious problem even when alternative chemistries are available to control resistant beetles. First, development of new insecticides is increasingly expensive, which affects their market price paid by growers. Grafius (1997) conducted an extensive four-year study of the economic impact of the Colorado potato beetle insecticide resistance on the Michigan potato industry. The estimated long-term increased cost ranged from $44 to $69/ha, with overall state-wide losses adding up to as much as $1.4 million per year. Secondly, available alternatives may be less convenient and more environmentally damaging than the failed insecticides they have to replace. For example, imidacloprid became ineffective for controlling Colorado potato beetles on two commercial farms in Southern Maine (Alyokhin et al. 2007). As a result, the growers were forced to replace a single application of imidacloprid in-furrow at planting with multiple foliar applications of oxamyl and abamectin. Both compounds have higher mammalian toxicity than imidacloprid, and foliar sprays increase worker exposure and the possibility of drift. Third, a threat of insecticide failure due to resistance development increases the already high real and perceived risks of potato farming.
RESISTANCE ASSAY METHODS
In the field, insecticide resistance can be observed as little or no reduction in the density of beetle populations and their damage to potato plants following insecticide application (assuming that other possible reasons, such as poor timing or malfunctioning spray equipment, could be ruled out). In the laboratory, resistance can be measured as an increase in the dose (LD50) or concentration (LC50) of the toxin required to kill 50% of the exposed population. LD50 values are used when the exact dose received by each tested insect is known (e.g., when a droplet of the known volume of the known concentration of insecticide solution is applied topically to an insect). LC50 values are used when the exact dose is not known (e.g., when an insect feeds on the leaf treated with the known concentration of insecticide solution, but the exact amount of ingested material is not measured). Bioassays normally consist of preparing serial dilutions of tested chemicals and then applying them to beetles originating from populations of interest. LD50 or LC50 values are then determined using probit analysis. Resistance ratios are often calculated as the ratio of LD50 or LC50 value obtained for a resistant population to the LD50 or LC50 value obtained for a control population that is known to be susceptible to the tested chemical. Discriminating doses or concentrations (e.g., the concentration that kills <5% of beetles from resistant populations but >90% of susceptible beetles) can also be determined from laboratory bioassays for quick identification of resistant beetles (Heim et al. 1990; Bishop and Grafius 1991).
Because differences in susceptibility to insecticides may be considerable among Colorado potato beetle life stages, LD50 or LC50 values and resistance ratios may differ depending on which life stage was assessed (Silcox et al. 1985; Zehnder 1986; Zehnder and Gelernter 1989; Wierenga et al 1996; Zhao et al. 2000). For example, imidacloprid resistance ratios differed by almost an order of magnitude when measured in 2nd instar larvae compared to adults (Zhao et al. 2000). As a result, susceptibility studies performed for one life stage may not accurately reflect susceptibility at a population level (Pourmirza 2005), nor do they necessarily accurately predict insecticide performance in the field.
Different studies rely on different exposure methods in their bioassays. For example, Boiteau et al. (1987), Argentine et al. (1989a), Ioannidis et al. (1991), Zhao et al. (2000), and Mota-Sanchez et al. (2006) applied solutions of technical grade insecticides in acetone topically to adult abdomens. Argentine et al. (1989a) also applied similar solutions to the third dorsal abdominal segment of fourth instars, while Baker et al. (2007) – to the abdomens of early second instars. Heim et al. (1990) and Pourmirza (2005) placed first instars on paper disks treated with solutions of commercial insecticide formulations in acetone. Bishop and Grafius (1991) and Mowry and Sandvol (1995) placed adults on paper disks treated with solutions of commercial insecticide formulations in distilled water. Whalon et al. (1993) fed early second instars on insecticide-treated potato foliage. Olson et al. (2000) fed first instars on artificial diets mixed with serial dilutions of tested chemicals. Harris and Svec (1981) and Alyokhin et al. (2006) applied commercial insecticide formulations to potted potato plants grown in greenhouses. Pourmirza (2005) dipped adults in water solutions of commercial phosalone concentration or placed them in glass jars coated with acetone solutions of the same compound. Mota-Sanchez et al. (2006) injected solutions of technical grade imidacloprid in dimethyl sulfoxide through the membrane that connects second and third adult abdominal segments. Variation in assay methodology often complicates the comparability of different studies, and some standardization would probably be helpful. However, it is to a certain degree unavoidable because of the diversity of properties and modes of action of different insecticides and differing objectives of research.
It is important to realize that numerical increase in LD50 or LC50 values does not always mean noticeable decline in insecticide efficiency in the field. For example, an increase of 15 to 20-fold in LD50 in laboratory assays was present before reduced control with neonicotinoid insecticides was noticed in the field in Michigan (Grafius unpublished). First, label application rates are usually conservative enough to kill all but highly resistant individuals. Secondly, data resulting from bioassays are usually highly variable, and outliers may inflate the means. Nevertheless, laboratory investigations provide valuable insight into resistance status of populations in question. Several studies directly confirmed subjective grower assessment of poor insecticide performance in their fields by the results of laboratory bioassays (Boiteau et al. 1987; Stewart et al. 1997; Alyokhin et al. 2007). Also, bioassays may detect early shifts in resistance levels before frequency of resistant beetles in a given population becomes apparent in the field.
PHENOTYPIC CHARACTERISTICS OF RESISTANT BEETLES
Variation in assay methodology often complicates the comparability of different studies, and some standardization would probably be helpful. Resistance levels vary greatly among different populations (Forgash 1985; Boiteau et al. 1987; Mowry and Sandvol 1995; Stewart et al. 1997; Olson et al. 2000) and between beetle life stages (Silcox et al. 1985; Zehnder 1986; Zehnder and Gelernter 1989; Pourmirza 2005), but in some cases could be very high. For example, Harris and Svec (1981) detected more than 1,600-fold increase in the LD50 of carbofuran in the field population from Quebec. Similarly, Ioannidis et al. (1991) observed more than 2,000-fold decrease in susceptibility to carbofuran and more than 1,000-fold decrease in susceptibility to azinphosmethyl in some Michigan populations. Such numbers definitely indicate a considerable resistance problem. However, high resistance ratios are not required to identify a population as resistant. For instance, a field population that could no longer be controlled by imidacloprid in the study by Alyokhin et al. (2007) showed only 30-fold increase in LC50 based on the results of larval laboratory bioassays. Numeric outcomes of bioassays are very dependent on the methodology (Pourmirza 2005), and their absolute values should be interpreted with some caution. All in all, there is no doubt that some Colorado potato beetle populations are capable of developing very high levels of insecticide resistance.
In addition to their ability to withstand insecticides, resistant beetles may differ from their susceptible conspecifics in other ways. Because of pleiotropic effects of resistant alleles, insecticide-resistant insects usually have reduced relative fitness in the absence of insecticides (Crow 1957). The Colorado potato beetle is no exception. Beetles resistant to a variety of chemicals have been shown to lay fewer eggs, have lower rates of egg hatch, and develop more slowly compared to susceptible beetles (Argentine et al. 1989b; Trisyono and Whalon 1997; Alyokhin and Ferro 1999c; Baker et al. 2007). Alyokhin and Ferro (1999c) reported that overwintering mortality was twice as high for the beetles resistant to Bacillus thuringiensis subsp. tenebrionis Cry3A delta-endotoxin (Bt) as for the susceptible beetles. Also, Baker and Porter (2008) saw a significant drop in imidacloprid resistance following winter diapause, suggesting lower survival of resistant individuals. Alyokhin et al. (2006) recorded increased mortality of the first instars in a different imidacloprid-resistant population. Alyokhin and Ferro (1999c) observed a 40% drop in the number of copulations secured by Bt-resistant males competing for the same females with susceptible males, but there was no difference in fertilization success or copulation frequency on untreated foliage between imidacloprid-resistant and imidacloprid-susceptible beetles (Baker et al. 2008). Flight activity of Bt-resistant beetles fed on untreated non-transgenic foliage was notably reduced compared to susceptible beetles (Alyokhin and Ferro 1999d), and so was the sprinting speed of imidacloprid-resistant beetles (Baker et al. 2007). Some of the resistance costs appear to be persistent, but others might decline over time, presumably due to allelic substitution or addition of modifiers (Baker et al. 2007).
Another reason why resistant populations might differ phenotypically from susceptible populations is genetic bottleneck. Insecticide applications may eliminate susceptible genotypes, thus reducing genetic variability of the surviving population. Resistant founders may possess random characteristics that are genetically determined, but not directly influenced by resistant alleles. For example, Benkovskaya et al. (2006) observed a much higher frequency of phenotypes with certain spot patterns on their heads, pronota, and elytra in the populations of insecticide-resistant beetles compared to the populations of susceptible beetles. An overall diversity of spot patterns decreased over the ten-year period, presumably due to insecticide selection pressure. Inbreeding depression may also contribute to the decreased relative fitness of resistant insects, although most studies also use highly inbred laboratory strains as susceptible controls.
MECHANISMS OF RESISTANCE
Known mechanisms of Colorado potato beetle resistance to insecticides include enhanced metabolism involving esterases, carboxylesterases and monooxigenases, target site insensitivity, as well as reduced insecticide penetration and increased excretion (Rose and Brindley 1985; Argentine et al. 1989a; Ioannidis et al. 1991; Ioannidis et al. 1992; Wierenga and Hollingworth 1994; Anspaugh et al. 1995; Zhu et al. 1996; Lee and Clark 1998; Clark et al. 2001). There is also some evidence of behavioral resistance (Hoy and Head 1995; Alyokhin and Ferro 1999d). Resistance mechanisms may be highly diverse even within a relatively narrow geographical area (Ioannidis et al. 1991).
Cytochrome P450 is involved in the metabolism of internal hormones and lipids in insects, and also participates in the metabolism of xenobiotic compounds such as pesticides (Feyereisen 2005). In the Colorado potato beetle, the P450 dependent monooxygenase system is the most common mechanism of resistance across many populations from diverse geographical origins.
In vivo, pre-treatment by an oxygenase inhibitor piperonyl butoxide (PBO) reduced resistance of adult beetles resistant to azinphosmethyl (Ahammad-Sahib et al. 1994), carbofuran and carbaryl (Rose and Brindley 1985), fenvalerate (Soderlund et al. 1983; Silcox et al. 1985; Harris and Turnbull 1986), permethrin (Silcox et al. 1985), and in 4th instar larvae reduce the resistance to abamectin (Yoon et al. 2002). Similarly, imidacloprid-resistant beetles from Long Island, New York showed a reduction in resistance from 300-fold without PBO treatment to 108-fold with PBO treatment (Mota-Sanchez et al. 2006). The insect metabolism of imidacloprid leads to the production of an olefin metabolite that is less toxic to the insect than the parent compound (Mota-Sanchez 2003). This phenomenon has been observed in both susceptible and resistant strains, but to a greater extent in the resistance strains.
In vitro, incubation of different substrates with microsomes confirmed that resistance due to monooxygenase activity was present in resistant strains from Long Island, New York and Michigan (Ahammad-Sahib et al. 1994). Monooxygenase activity was observed in a variety of different substrates including oxidation of NADPH, O-demethylation, of p-nitrianisole, N-demethylation of aminopyrine and epoxidation of aldrin. A key finding was that monooxygenase activity against aminopyrine was largely confined to the microsomal fraction of homogenates and was more active in the larval gut than in the fat body. Beetle strain resistant to azinphos methyl also exhibited 4-fold higher metabolism of the substrates ethoxyresorufin and pentoxyresorufin (Wierenga and Hollingworth 1994). Increased metabolism and microsomal P450 levels were associated with resistance of 4th instar larvae to abamectin (Gouamene-Lamine et al. 2003). Pre-treatment with PBO suppressed metabolism of 14C-imidacloprid in resistant beetles, providing further evidence of the role of PBO in suppression of P450 metabolism (Mota-Sanchez, unpublished data). Similarly, the main mechanisms of resistance to azinphosmethyl and permethrin have been identified as increased monooxygenase and arylesterase activity against azinphos methyl and permethrin (Argentine et al. 1989a). Enhanced carboxylesterase activity also contributed to beetle resistance to permethrin (Argentine et al. 1995; Lee and Clark 1996). In addition, gluthione-S-transferase activity was elevated in the Long Island strain resistant to azinphosmethyl, as well as in the Michigan strain resistant to azinphosmethyl, permethrin and carbofuran (Ahammad-Sahib et al. 1994).
Target site insensitivity is another important mechanism of beetle resistance to insecticides. Altered acetylcholine esterase plays a critical role in resistance to organophosphates and carbamates (Ioannidis et al. 1992; Wierenga and Hollingworth 1993; Stankovic et al. 2004). Beetles resistant to azinphosmethyl contained two mutations in the AChE (S291G and R30K), which made the enzyme less sensitive to azinphosmethyl and carbofuran (Kim and Clark 2002; Kim et al. 2006). In the strain resistant to carbofuran, the presence of two mutations (I392T and S291G) did not result in resistance, but the presence of just one (S291G) conferred high resistance to carbofuran and medium resistance to azinphosmethyl (Kim et al. 2007). A strain resistant to azinphosmethyl because of an altered acetylcholine esterase had a reduced substrate affinity for acetylthiocholine and azinphosmethyl oxon in comparison with a susceptible strain (Clark 1997).
Resistance due to the changes in acetylcholine esterase may be selective. For instance, Wierenga and Hollingworth (1993) found that while one resistant strain was highly insensitive to arylcarbamates, another strain with the same affected enzyme was highly insensitive to organophosphates, but not arylcarmabates. Interestingly, acetylcholinesterase alteration made yet another resistant strain more sensitive to α-chaconine, a glycoalkaloid present in potatoes and an inhibitor of acetylcholine esterase (Wierenga and Hollingworth 1992). In addition, altered acetylcholine esterase also increased sensitivity to tomatine, a glycoalkaloid found in tomatoes (Wierenga and Hollingworth 1992).
Target site changes are not limited to acetylcholine esterase. In a permethrin-resistant Colorado potato beetle strain, a single base pair mutation (C to T) resulted in an amino acid change (leucine to phenylalanine, L1014F) in an α-subunit of the sodium channel. That mutation was the major factor responsible for nerve insensitivity in Colorado potato beetles when exposed to permethrin (Lee et al. 1999, Kim et al. 2005). Also, nerve recording suggested that insensitivity at the target site may be one of the mechanisms of beetle resistance to imidacloprid (Tan et al. 2005). However, although mutation in the nicotinic acetylcholine receptor was responsible for resistance of the brown planthopper to imidacloprid (Liu et al. 2006), no mutation has been reported in the alpha subunits of the target site responsible for resistance in the Colorado potato beetle. Additionally, no differences were found in binding receptors of 3H-imidacloprid in resistant and susceptible beetles (Nauen and Denholm 2005).
Reduced penetration and increased excretion appear to be less important in reducing Colorado potato beetle sensitivity to insecticides. Nevertheless, they also have their role to play. Argentine et al. (1994) found that these mechanisms may act together with enhanced metabolism and target site insensitivity to reduce toxicity of azinphosmethyl. Reduced penetration and increased excretion were also important in carbaryl resistant beetles (Rose and Brindley 1985). In addition, rapid excretion has been identified as a mechanism to remove imidacloprid (Mota-Sanchez 2003) and glycoalkaloids (Krishnan et al. 2007) from the insect body. In vivo distribution and metabolism studies using [14C] imidacloprid suggested that the tolerance observed in a resistant population from Long Island, New York was primarily due to increased excretion of the parent compound (Olson 2000).
Behavioral resistance has been studied in the Colorado potato beetles resistant to Bt. Alyokhin and Ferro (1999d) reported that the ingestion of the toxin significantly increased flight activity in the resistant strain, suggesting that physiological resistance was probably reinforced by the behavioral escape from the toxic environment. Such a response was displayed both by beetles continuously fed on transgenic plants and by beetles fed on regular plants before feeding on transgenic plants. A similar effect was reported by Hoy and Head (1995), who observed that Colorado potato beetle larvae with higher physiological resistance to Bt compared to susceptible larvae were also more behaviorally responsive, moving away from the foliage treated with the higher toxin concentrations.
GENETICS OF RESISTANCE
Genetic analyses of resistance in the Colorado potato beetle have been carried out on carbamates, organophosphates, pyrethroids, Bacillus thuringiensis subsp. tenebrionis delta-endotoxin, and neonicotinoids. There have been four basic designs used to elucidate the genetics of insecticide resistance, often in combination. A positive response to selection can show in the simplest sense the presence of additive genetic variance for resistance (Argentine and Clark 1990; Ioannidis et al. 1992; Whalon et al. 1993; Rahardja and Whalon 1995; Trisyono and Whalon 1997; Miyo et al. 1999). Crosses between resistant and susceptible strains with backcrosses of hybrids to susceptibles have been used to measure dominance, sex linkage and single-locus vs. polygenic determination of resistance (Argentine et al.1989a; Heim et al. 1992; Ioannidis et al. 1992; Rahardja and Whalon 1995; Baker et al. 2007). Quantitative genetic estimates of heritability have been used to show potential for subsequent resistance evolution (Hoy and Head 1995; Miyo et al. 1999; Baker et al. 2007). Finally, linkage mapping (Hawthorne 2003) and cDNA sequencing (Zhu and Clark 1995) have been used to identify specific genes associated with resistance.
Several factors pose challenges for genetic analyses of resistance. The different assay techniques described above pose challenges in terms of validity and reliability. Direct application of a dissolved technical product is the least variable and most repeatable mode of application, but may not act in the same way as an ingested toxin. Pesticides can have very different effects on different age classes (see above). Parent-offspring regression, one of the simplest methods of measuring additive genetic variation, is not available in its usual form, because scoring the trait usually involves mortality. While an assay is usually qualitative, registering survival or mortality, the trait of interest is quantitative, i.e., probability of death. The quantitative genetic analysis of threshold traits such as sex-ratio of offspring (Bulmer and Bull 1982) can be applied to analysis of resistance, though there is still difficulty in using an assay that might kill off the parentals of a subsequent generation. One way of dealing with this is to use the LD50 of entire families as a measure of resistance, and compare resistance of offspring with grandchildren, using LD50 of full sibling families as a proxy for parental resistance (Baker et al. 2007). A half-sib design, where several males are mated to at least two females each and the variance within dams is compared to that among sires, can avoid some of these challenges to determining the heritability of resistance (Kearsey and Pooni 1996). Finally, costs of resistance can reduce genetic variation for resistance from one generation to the next, and reduce the effectiveness of parent offspring regression. This is seen most clearly in resistance genes with high associated fitness costs, such as imidacloprid (Baker et al. 2007).
Laboratory selection is expected to act differently than selection in the field. The strong selection exerted by highly toxic insecticides in the field is expected to favor dominant, single locus mutations conferring resistance, while the weaker selection possible with the relatively small populations sustainable in laboratory experiments will reveal smaller additive effects from existing genetic variation (McKenzie 2000). However, both laboratory selection experiments and crosses using field collections have been carried out using the same chemicals and those predictions have not generally been met (Table 3). For example, carbofuran resistance was examined genetically twice, once using laboratory selection (following selection in the field; Ioannidis et al. 1992) and once using crosses from a field-resistant population (Heim et al. 1992). The laboratory selected population had a higher resistance ratio, more dominant expression, and a closer match to single-locus predicted mortality in the backcross. In fact, the two studies with the highest degree of dominance were laboratory selection studies (Ioannidis et al. 1992; Rahardja and Whalon 1995). Resistance was never recessive, and the overall average value of D (after Stone 1968; where -1 is purely recessive, 0 is purely additive, and 1 is purely dominant) in the studies analyzed was (M±SD) 0.21±0.29, reflecting slight partial dominance. Most studies found evidence of more than one locus contributing to resistance, including all studies using field-resistant populations (Table 3).
| Treatment |
Generations1 |
Loci |
Sex-linked |
Resistance Ratio |
Dominance5 |
Source |
|
Carbofuran |
4 |
1 |
No |
576 |
0.62 |
Ioannidis et al. 1992 |
|
Carbofuran |
Direct cross |
>1 |
No |
69 |
0.26 |
Heim et al. 1992 |
|
Azinphosmethyl |
Direct cross |
>1 |
No |
435 |
0.26 |
Argentine et al. 1989a |
|
Permethrin |
Direct cross |
>1 |
Yes |
55 |
Recessive |
Argentine et al. 1989a |
|
Esfenvalerate |
Direct cross |
1 or >1 |
Yes |
20-21 |
Semi-recessive |
Heim et al. 1992 |
|
Esfenvalerate |
4 |
>1 |
Yes |
18 |
-0.26 |
Miyo et al. 1999 |
|
Permethrin |
Direct cross |
n.a. |
Yes |
41-47.5 |
Semi-recessive |
Follett et al. 1993 |
|
Imidacloprid |
Direct cross |
>1 |
No |
100.8 or 13.23 |
-0.23 or -0.106 |
Zhou et al. 2000 |
|
Imidacloprid |
1, 3 |
>1 |
No |
6.6-21.84 |
0.15-0.18 |
Baker et al. 2007 |
|
Btt |
29 |
>1 |
No |
293 |
0.77 |
Whalon et al. 1993, Rahardja and Whalon 1995 |
|
Abamectin |
7 |
n.a. |
No |
15-23 |
0.29 |
Argentine and Clark 1990 |
|
Abamectin |
7, 62 |
n.a. |
No |
15-23 |
0.29, 0.19 |
Argentine and Clark 1990 |
1 Either the number of generations of selection if selection was carried out prior to crossing, or ‘Direct cross’ to signify either immediate crosses between and within resistant and susceptible populations, or backcrossing.
2 Two separate populations were selected in this study.
3 The larger ratio was observed when resistance was assayed in adults, the smaller in 2nd-instar larvae.
4 E The smaller ratio was in 2nd-instars in Massachusetts in 1999, the higher ratio was from a Resistant field in Maine in 2005.
5 Dominance of the LD50 or LC50 was calculated as in Stone (1968). It ranges from -1 (purely recessive) to 1 (purely dominant), with 0 reflecting purely additive inheritance. When not reported it was calculated from the reported RR, SS, and RS LD50‘s, in the autosomal cases, and described qualitatively (except in Miyo et al. 1999) in sex-linked characters.
6 The more recessive value was measured in adults, the more additive one in larvae.
Imidacloprid has been a recent focus of research, due to its heavy use by the potato industry for control of Colorado potato beetles since 1995. While no genetic mapping of imidacloprid resistance has been carried out, simple crosses have shown that resistance is autosomal, with partially recessive or partially dominant inheritance observed in different studies (Table 3). Dominance can evolve (Bourguet et al. 2000), and the different values measured could have a significant effect on resistance evolution, but it is not possible to determine the significance of differences between the earlier result looking at a Long Island NY population (Zhao et al. 2000) and later results from Massachusetts and Maine populations (Baker et al. 2007), because the two studies looked at different age classes. The earlier study examined dominance in adults (and found different values depending on the day of scoring) and the later study looked at larval survival. Dominant genetic control is a challenge for resistance management because it preserves resistant alleles found in hybrid offspring, which is where most resistance alleles are found when resistance is rare. Once resistance is common this is less of a problem, because a smaller proportion of resistance alleles are found in heterozygotes. However, the greater the dominance of resistance, the higher application rate will be needed to control a genetically heterogenous population. It remains to be seen how the genetic underpinnings of resistance in different geographical locations varies. Dominance of fitness costs has a significant effect on resistance evolution in evolutionary models (Carriere et al. 2005). Unfortunately, the only measures thus far on dominance of fitness costs found a fecundity cost to be recessive and a hatch time cost to be additive (Baker et al. 2007).
Resistance to imidacloprid is likely polygenic. In two studies, observed mortality of backcross progeny was significantly different from predicted mortality using a single gene model (Zhao et al. 2000; Baker et al. 2007). Multiple mechanisms of resistance, presumably under control of different loci, have been indicated in several studies. Synergism studies (Zhao et al. 2000; Olson 2000; Mota-Sanchez et al. 2006) have shown a partial role for mixed-function oxydases, and a receptor sensitivity mechanism has also been described (Tan et al. 2008).
Quantitative genetic analyses can add another dimension to our understanding of resistance evolution. Slopes of dose-response curves are limited in their ability to measure relative and absolute amounts of genetic variability for resistance (McKenzie 2000). Heritabilities are valid only within the population measured, though different populations can be compared in a laboratory setting. They are also snapshots in time, because novel mutations can generate new genetic variance. However, they are the best measure of current additive genetic variance. There have been three estimates of heritability of resistance in the Colorado potato beetle. A full-sib design reported a heritability of 0.7 for tolerance to B. thuringiensis delta-endotoxin (Hoy and Head 1995). A half-sib design reported a heritability of 0.45 (but the value was not significantly different from 0) for tolerance to esfenvalerate (Miyo et al. 1999). A study using parent-offspring regression reported a heritability of 0.86 for imidacloprid tolerance (Baker et al. 2007). High variance in insecticide resistance systems is usually interpreted as reflecting a potential to rapidly lose susceptibility. However, high heritabilities, and high levels of additive variation, can also reflect opposing selection on resistance due to fitness costs of resistance, as each generation experiences the opposing forces of selection due to insecticide application and costs of resistance.
Molecular genetic analyses of resistance have been carried out on pyrethroids and azinphosmethyl, an organophosphate. The sex linkage and polygenic effects on permethrin resistance observed in earlier studies was confirmed using Quantitative Trait Locus (QTL) mapping (Hawthorne 2003), and genotyping tests for the locus with the strongest effect have been developed (Kim et al. 2005). A mutational change in the acetylcholine receptor of azinphosmethyl resistant potato beetles was sequenced (Zhu and Clark 1997). Molecular genetic studies should aid in identifying mechanisms of resistance, and should also identify when individual resistance mutations are shared by different populations and contribute to resistance against different active ingredients.
RESISTANCE MANAGEMENT
Reducing insecticidal pressure on pest populations is a commonly proposed strategy to delay evolution of resistance (McGaughey and Whalon 1992; Tabashnik 1994; Caprio 1998, Carrière and Tabashnik 2001). Selection towards insecticide resistance could be alleviated by replacing at least some of the sprays by non-chemical control techniques. Crop rotation for the Colorado potato beetle control had been first recommended as early as 1872 (Bethune 1872), and since then proved to be a good control strategy (Casagrande 1987). At the rotated field, peak density of the beetle egg masses could be 5-10% of that of the non-rotated field (Lashomb and Ng 1984; Wright 1984). Unfortunately, high beetle mobility necessitates a considerable separation between the rotated fields to maximize efficiency of this technique (Weisz et al. 1994, 1996; Hough-Goldstein and Whalen 1996). Still, crop rotation remains the single most important cultural control against the Colorado potato beetle.
Other cultural practices that show potential to reduce beetle damage include manipulation of planting time (Weber and Ferro 1994), use of trap crops (Weber and Ferro 1994; Hoy et al. 1996), mulches (Zehnder and Hough-Goldstein 1990; Stoner 1993; Brust 1994), plastic-lined trenches along field borders (Misener et al. 1993; Boiteau et al. 1994), and destruction of overwintering habitats (Milner et al. 1992). Natural enemies can also have a substantial negative impact on beetle populations (Hough-Goldstein et al. 1993; Ferro 1994), but because of the high Colorado potato beetle fecundity they usually cannot suppress beetle populations below economically damaging levels. Except for crop rotations, the overall feasibility of alternative control techniques is usually not sufficient to allow their large-scale adoption in commercial production.
Host plant resistance is a potentially valuable tool for managing Colorado potato beetles that could reduce the number of insecticide applications. Unfortunately, resistant cultivars are not currently available. Although numerous Solanum spp. have resistance to insects, potato breeding is complicated by the potato’s tetraploidy and these properties have not been transferred into commercially desirable cultivars (Grafius and Douches 2008). Genetically modified potatoes expressing Bacillus thuringiensis delta-endotoxin that is toxic to the Colorado potato beetle were registered and sold in the U.S. from 1995–2000, but were discontinued in response to consumer concerns about genetically modified crops (Grafius and Douches 2008).
Rotation between insecticides with different modes of action is one of the most commonly recommended resistance management techniques (Insecticide Resistance Action Committee 2008; National Potato Council 2008). This approach may work particularly well when growers practice integrated pest management and no insecticide applications are made unless beetle densities exceed economic thresholds. Unfortunately, Colorado potato beetles are commonly resistant to multiple chemicals belonging to different insecticide classes and resistance mechanisms may be highly diverse even within a relatively narrow geographical area (Ioannidis et al. 1991). Also, there is a considerable variation in toxicity to Colorado potato beetles of different insecticides belonging to the same chemical class (Forgash 1985; Boiteau et al. 1987; Alyokhin et al. 2006; Mota-Sanchez et al. 2006). All this makes selection of appropriate rotation chemicals a rather challenging task. Furthermore, beetle populations on most commercial farms have a long history of exposure (and subsequent resistance) to a variety of chemical classes. Although frequency of alleles conferring resistance to a given insecticide is likely to decline after a grower stops using it, it will still stay elevated compared to unexposed populations. As a result, there will likely be reinvigoration of resistance development to at least some of the older chemicals following their re-introduction to the rotation sequence (Alyokhin et al. 2006). Knowing the past insecticide exposure and current susceptibility to individual insecticides for a particular Colorado potato beetle population is important for achieving good levels of its suppression. Simple replacement of one class of chemicals with another is not necessarily going to work.
Spatial refuges can be created by leaving a proportion of the field (usually about 20%) untreated with insecticides to support a population of susceptible individuals sufficient to curtail mating between resistant individuals (Whalon and Ferro 1998). Success of this approach depends on the existence of a large population of susceptible individuals and a significant gene flow between resistant and susceptible Colorado potato beetle populations. Mating between resistant and susceptible beetles is likely to be encouraged by high beetle mobility (especially when crop rotation is practiced) and an extended period of sexual activity characteristic of this species. When present, behavioral avoidance of toxic environments by resistant beetles may further increase an outflow of resistant alleles from the main crop into refuges (Alyokhin and Ferro 1999d), where a homozygously resistant population is unlikely to establish because of its reduced relative fitness (Trisyono and Whalon 1997; Alyokhin and Ferro 1999c). Gene flow, and refuge success, will be limited by differences in phenology in treated and untreated areas. Incomplete resistance, leading to longer development time in transgenic Bt crops has been identified as an obstacle to gene flow and refuge success in the cotton pest pink bollworm moth, Pectinophora gossypiella (Liu et al. 1999). A similar effect on phenology was seen in the Colorado potato beetle populations on imidacloprid treated and untreated sides of a single field. In that study the peak in large (third or fourth instar) larval density was delayed by 30 days in the first summer generation (Baker et al. 2001). If gene flow during the summer generations is hampered by changes in phenology, the bulk of gene flow must take place in the populations emerging from diapause in the spring.
When beetles aggregate in overwintering sites outside of the field (Weber and Ferro 1993), this will also contribute to gene flow between resistant and susceptible populations. After diapause termination, a significant proportion of post-diapause beetles mate within or near overwintering sites (Ferro et al. 1999). Since overwintering survivorship may be higher for susceptible beetles than for resistant beetles (Alyokhin and Ferro 1999c; Baker and Porter 2008), and susceptible males may secure more per capita copulations than resistant males (Alyokhin and Ferro 1999c), there is an increased probability that a surviving post-diapause resistant female will mate in the spring with a susceptible male. Sperm from the spring mating takes almost complete precedence over the sperm from the pre-diapause mating (Baker et al. 2005). As a result, frequency of resistant homozygotes in post-diapause beetle populations would be lower than in the populations of summer-generation beetles. Orienting untreated areas parallel to overwintering sites will encourage susceptible beetles from refuges and resistant beetles from the main crop to overwinter in the same sites (Ferro et al. 1999).
Refuges could be created by leaving blocks of untreated plants adjacent to the treated fields, by leaving untreated strips within the larger fields (Whalon and Ferro 1998), or by using perimeter sprays or at-planting treatments with a block of untreated rows in the middle of the field (Dively et al. 1998; Blom et al. 2002). Colonization of potato crops by insect pests starts at field edges, and then progresses towards the field center (Voss and Ferro 1990; Weber and Ferro 1993; Boiteau et al. 1994; DiFonzo et al. 1996). Therefore, perimeter treatment provides protection to the untreated refuge because treated rows serve as barriers to the pests colonizing potato fields. However, leaving too narrow of a treated perimeter may result in inadequate crop protection (Blom et al. 2002). At the same time, too wide a perimeter may de-facto eliminate the refuge because susceptible colonizers perish while trying to cross the treated areas.
Although the idea of a spatial refuge is very popular in the academic community, most commercial growers perceive this approach as risky and are reluctant to implement it on their farms because of the high value of the potato crop and the difficulty often encountered controlling Colorado potato beetles. Delaying resistance development is a somewhat abstract and distant benefit, while reduced yield due to beetle damage is a real and present loss. When leaving a refuge is a legal requirement, as is the case with transgenic corn and cotton plants expressing Bt-toxins in their foliage, the growers normally insist on treating it with chemicals that have a different mode of action. Depending on the chemicals used and the frequency of their application, this may effectively cancel the refuge by killing most (if not all) of its inhabitants.
It is highly unlikely that there is a silver-bullet solution to the problem of insecticide resistance in the Colorado potato beetle. By itself, none of the discussed techniques will be successful in preserving efficiency of crop protection chemicals. Resistance development is a dynamic evolutionary process that could be likened to an arms race between humans coming up with new toxins and ways of their deployment and pests developing new adaptations (Denholm and Rowland 1992). Only a truly integrated approach to pest management will ensure long-term success in chemical pest control (which is another way of saying “will prevent resistance development”). To continue the military analogy, the same three basic principles that apply to any hostile encounter also apply to suppression of the Colorado potato beetle populations. First, it is essential to know and understand the enemy. Secondly, it is necessary to use a multiple attack strategy. Relying on a single tactic is usually doomed to failure, even if this tactic is sound in general (just think of an army that consists exclusively of tank brigades, without infantry, aviation, or engineering corps). Third, it is important to strike hard, but stop once the adversary ceases hostilities and is ready to surrender.
In practical terms, an on-farm resistance management plan should include the following elements:
- Supplementing insecticides with non-chemical control methods (particularly crop rotations).
- Alternating insecticides with different modes of action.
- Using economic thresholds when making decisions about spraying. Trying to kill all the beetles with insecticides usually results in killing all susceptible beetles, while resistant beetles survive and quickly build up their numbers.
- Leaving untreated refuges for susceptible beetles. Economic thresholds cannot be used when insecticide is applied at planting time in furrow or as a seed treatment. Therefore, spatial refuges are required to maintain populations of susceptible beetles.
- Using full label rate of insecticides. Because resistance is usually incompletely dominant, sufficiently high dose of a toxin will kill the beetles that are heterozygous at the resistant allele.
- Monitor for the signs of decreasing insecticide efficiency.
Similar to other situations, an ounce of prevention is likely to be worth a pound of cure when it comes to resistance management. In theory, reduced relative fitness of resistant beetles should cause populations to revert toward susceptibility in the absence of selection pressure (Denholm and Rowland 1992). Indeed, Rahardja and Whalon (1995) reported that under laboratory conditions the ratio of Colorado potato beetle resistance to Bt decreased from 200 to 48-fold in 12 generations without selection. However, it remained stable afterwards. The last generation tested in that study (17th after removal of selection pressure) still was significantly more resistant than unselected beetles. Similarly, beetles on a commercial farm remained tolerant to imidacloprid for four years after the grower discontinued using neonicotinoids (Alyokhin 2007), despite the resistance-related reduction in relative fitness in that population (Baker et al. 2007). It is possible that in the absence of a refuge, susceptible genotypes are largely eliminated from the population. As a result, the remaining genetic variation is not sufficient to provide a quick reversion to susceptibility, even when susceptible genotypes have a higher relative fitness. This strongly supports taking a proactive approach to resistance management. It will be too late to implement a management plan after an insecticide has failed in the field.
CURRENT AND FUTURE CHALLENGES
In his now classical treatise, Casagrande (1987) described the long history of the Colorado potato beetle control as “135 years of mismanagement.” The insecticide resistance crisis that was unfolding at that time was one of the primary reasons for such an unflattering definition, but the picture remains largely the same. Despite all the scientific and technological advances, the Colorado potato beetle continues to be a major threat to potato production. Unfortunately, there are compelling reasons to believe that in the foreseeable future, insecticide resistance in this insect will remain a major challenge to the pest control practitioners.
First, our understanding of the Colorado potato beetle biology is still incomplete. In some cases, recommendations on resistance management are developed based on studies of as little as one resistant strain, often selected under laboratory conditions. Although such an approach is often valid, resistance mechanisms may be diverse even within a relatively limited geographic area (Ioannidis et al. 1991). Therefore, information obtained in one location will not necessarily apply to other locations where resistance becomes a problem. In other cases, recommendations are made based on mathematic models, either specifically developed for the Colorado potato beetle/potato system (Follett et al. 1993, 1995), or borrowed from other systems (e.g., Baker et al. 2007). Again, this could be a valuable approach if an appropriate model is selected. However, model assumptions cannot be taken for granted and should be verified using experimental techniques. For example, Zhao et al. (2000) and Baker et al. (2007) found evidence of polygenic inheritance of resistance to imidacloprid in the field Colorado potato beetle while most theoretical models advocating high dose – refuge approach to insecticide resistance management assume that resistance is controlled by a single gene.
Secondly, some known aspects of the Colorado potato beetle life history complicate resistance management. For instance, about 25% of recently emerged summer generation beetles stay close to the place of their larval development until their reproductive system is mature (Alyokhin and Ferro 1999a). Therefore, a significant proportion of resistant beetles developing to adulthood on treated plants will mate with each other and leave homozygously resistant offspring. Even when resistant females previously mated to resistant males mate to susceptible males, up to half of their offspring will still be homozygously resistant (Boiteau 1988a; Alyokhin and Ferro 1999b). As a result, only complete cessation of mating between resistant beetles will remove all resistant homozygotes from the population. Clearly, that could hardly happen under field conditions.
High Colorado potato beetle mobility is also somewhat of a mixed blessing when it comes to resistance management. On one hand, it encourages gene flow between refuges and the main treated crop. On the other hand, it may increase spread of resistance among geographically isolated fields. Grafius (1995, 1997) suggested that unusually warm spring weather encouraged early beetle emergence from diapause in the spring of 1991 in Michigan. Because beetles were out of the soil before emergence of potato crop, many of them engaged in long-distance migratory flight in search of hosts. As a result, resistance became a problem in previously unaffected areas, Colorado potato beetle control costs rose by half, and crop losses to beetle damage went from virtually zero to as high as 12.2% (Grafius 1997).
Extended diapause is another life history trait potentially complicating resistance management (Whalon and Ferro 1998). Efficiency of annual crop rotation for reducing field colonization by overwintering adults will obviously be diminished when a portion of population remains dormant for two years. Furthermore, it will complicate insecticide rotations in a continuous potato crop. For example, some growers may want to alternate at-planting applications of systemic neonicotinoids with foliar applications of different chemicals during the following year. If substantial numbers of neonicotinoid-resistant beetles do not exit their diapause until the third year, efficiency of such an approach is compromised. Even if the field is rotated to a non-host crop in the third year, long-term dispersal of resistant beetles may export resistance to other fields (Grafius 1995). Selection for multiyear diapause in response to crop rotation on commercial farms has been reported for another leaf beetle, northern corn rootworm (Diabrotica barberi Smith & Lawrence) (Levine et al. 1992).
Third, prevailing grower attitude remains to be a barrier to wide-scale adoption of resistance management techniques. The US National Potato Council has recognized the importance of management of resistance for the Colorado potato beetle, diseases, and weeds, resulting in the development of educational materials that have been sent to growers through state potato organizations (National Potato Council 2008). Although there is a general acknowledgement of the problem, dealing with it remains low on an average grower’s list of priorities. Few potato farmers are willing to modify existing practices and suffer significant inconveniences or take on additional risks to delay Colorado potato beetle adaptation to insecticides. Instead, most of them hope that by the time when the current chemical of choice fails, the insecticide industry will come up with some new compound. A considerable education effort on part of the research and extension community is required before resistance management becomes as essential a part of good pesticide stewardship as preventing drift or using personal protection equipment.
Overcoming these challenges is not an easy task. However, it is crucial for sustainable potato production. The era of abundant and cheap broad-spectrum insecticides has come to an end. Development and registration of new insecticides is an increasingly complicated and costly process. At the same time, older chemistries are being lost to resistance or removed from the market because of environmental concerns. As a result, preservation of existing products becomes a progressively more important task for everybody involved in commercial agriculture. Insecticide susceptibility in pest populations should be treated as a valuable genetic resource (Hueth and Regev 1974; Bourguet et al. 2005), similar to drought tolerance or disease resistance in cultivated plants. Thus, its preservation needs to be taken extremely seriously by all interested parties. Excessive reliance on chemicals is a dangerous case of “putting all eggs in one basket,” with the fallacy of such an approach being repeatedly and expensively demonstrated throughout the history of pest control. It is essential that for the years to come we have a variety of techniques available for suppressing pest populations.
REFERENCES CITED
Ahammad-Sahib KI, RM Hollingworth, ME Whalon, PM Ioannidis and EJ Grafius. 1994. Polysubstrate monooxygenases and other xenobiotic-metabolizing enzymes in susceptible and resistant Colorado potato beetle. Pestic Biochem Physiol 49:1-12.
Alyokhin AV. 2007. Results of the 2007 Imidacloprid resistance survey in Maine populations of the Colorado potato beetle. Spudlines 45(3):3-4.
Alyokhin AV and DN Ferro. 1999a. Reproduction and dispersal of summer-generation Colorado potato beetle (Coleoptera: Chrysomelidae). Environ Entomol 28:425-430.
Alyokhin AV and DN Ferro. 1999b. Electrophoretic confirmation of sperm mixing in mated Colorado potato beetles (Coleoptera: Chrysomelidae). Ann Entomol Soc Am 92:230-235.
Alyokhin AV and DN Ferro. 1999c. Relative fitness of Colorado potato beetle (Coleoptera: Chrysomelidae) resistant and susceptible to the Bacillus thuringiensis Cry3A toxin. J Econ Entomol 92:510-515.
Alyokhin AV and DN Ferro. 1999d Modifications in flight and oviposition of Bt-resistant and Bt-susceptible Colorado potato beetles as a result of exposure to Bacillus thuringiensis subsp. tenebrionis Cry3A toxin. Entomol Exp Appl 90:93-101.
Alyokhin A, G Dively, M Patterson, D Rogers, M Mahoney and J Wollam. 2006. Susceptibility of imidacloprid-resistant Colorado potato beetles to non-neonicotinoid insecticides in the laboratory and field trials. Am J Potato Res 83: 485-494.
Alyokhin A, G Dively, M Patterson, C Castaldo, D Rogers, M Mahoney and J Wollam. 2007. Resistance and cross-resistance to imidacloprid and thiamethoxam in the Colorado potato beetle. Pest Manage Sci 63:32-41.
Anspaugh D, DG G Kennedy and RM Roe. 1995. Purification and characterization of a resistance-associated esterase from the Colorado Potato Beetle, Leptinotarsa- Decemlineata (Say). Pestic Biochem and Physiol 53:84-96.
Argentine JA and JM Clark. 1990. Selection for abamectin resistance in Colorado potato beetle (Coleoptera, Chrysomelidae). Pestic Sci 28: 17-24.
Argentine JA, JM Clark and DN Ferro. 1989a. Genetics and synergism of resistance to azinphosmethyl and permethrin in the Colorado potato beetle (Coleoptera, Chrysomelidae). J Econ Entomol 82: 698-705.
Argentine JA, JM Clark and DN Ferro. 1989b. Relative fitness of insecticide-resistant Colorado potato beetle strains (Coleoptera: Chrysomelidae). Environ Entomol 18:705-710.
Argentine JA, KY Zhu, SH Lee and JM Clark. 1994. Biochemical-mechanisms of azinphosmethyl resistance in isogenic strains of Colorado potato beetle. Pestic Biochem Physiol 48:63-78.
Argentine JA, SH Lee, MA Sos, SR Barry and JM Clark. 1995. Permethrin resistance in a near isogenic strain of Colorado potato beetle. Pestic Biochem Physiol 53:97-115.
Baker MB and AH Porter. 2008. Use of sperm precedence to infer the overwintering cost of insecticide resistance in the Colorado potato beetles (Coleoptera: Chrysomelidae). Agric Forest Entomol, in press.
Baker MB, SR Dastur, T Wong and BD Jaffe. 2008. Mating competition between Colorado potato beetles (Coleoptera: Chrysomelidae) resistant or susceptible to imidacloprid does not show cost of resistance. Ann Entomol Soc Am, in press.
Baker MB, DN Ferro and AH Porter. 2001. Management of a well established crop pest: Colorado potato beetle invasions on large and small scales. Biol Invasions 3: 295-306.
Baker MB, A Alyokhin, SR Dastur, AH Porter and DN Ferro. 2005. Sperm precedence in the overwintered Colorado potato beetles (Coleoptera: Chrysomelidae) and its implications for insecticide resistance management. Ann Entomol Soc Am 98:989–995.
Baker MB, A Alyokhin, AH Porter, DN Ferro, SR Dastur and N Galal. 2007. Persistence and inheritance of costs of resistance to imidacloprid in Colorado potato beetle. J Econ Entomol 100:1871-1879.
Benkovskaya GV, MB Udalov, AG Nikolenko and TL Leontieva. 2006. Temporal and toxicological dynamics in the cover spot patterns of the Colorado potato beetle in South Ural. Resist Pest Manage 15:13-15.
Bethune CJS. 1872. Report of the Entomological Society of Ontario for the year 1871. Hunter, Ross, Toronto.
Biever KD and RL Chauvin. 1990. Prolonged dormancy in a Pacific Northwest population of the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Can Entomol 122:175-177.
Bishop, BA and EJ Grafius. 1991. An on-farm insecticide resistance test kit for Colorado potato beetle (Coleoptera: Chrysomelidae). Am Potato J 68:53-64.
Bishop BA and EJ Grafius. 1996. Insecticide resistance in the Colorado potato beetle. In: P Jolivet and TH Hsiao. Chrysomelidae Biology, Volume 1. SBP Academic Publishing, Amsterdam.
Blom PE, S Fleischer and Z Smilowitz. 2002. Spatial and temporal dynamics of Colorado potato beetle (Coleoptera: Chrysomelidae) in fields with perimeter and spatially targeted insecticides. Environ Entomol 31:149-159.
Boiteau G. 1988a. Sperm utilization and post-copulatory female-guarding in the Colorado potato beetle, Leptinotarsa decemlineata. Entomol Exp Appl 47:183-187.
Boiteau G. 1988b. Control of the Colorado potato beetle, Leptinotarsa decemlineata (Say): learning from the Soviet experience. Bull Entomol Soc Can 20:9-14.
Boiteau G, RH Parry and CR Harris. 1987. Insecticide resistance in New Brunswick populations of the Colorado potato beetle (Coleoptera: Chrysomelidae). Can Entomol 119:459-463.
Boiteau G, Y Pelletier, GC Misener and G Bernard. 1994. Development and evaluation of a plastic trench barrier for protection of potato from walking adult Colorado potato beetles (Coleoptera: Chrysomelidae). J Econ Entomol 87:1325-1331.
Boiteau G, A Alyokhin and DN Ferro. 2003. The Colorado potato beetle in movement. Can Entomol 135:1-22.
Bourguet D, M Desquilbet and S Lemarie. 2005. Regulating insect resistance management: the case of non-Bt corn refuges in the US. J Environ Manage 76:210-220.
Bourguet D, A Genissel and M Raymond. 2000. Insecticide resistance and dominance levels. J Econ Entomol 93:1588-1595.
Brown AWA. 1951. Chemical control of insects feeding on plants. In: Insect Control by Chemicals. John Wiley, New York, pp. 574-667.
Brust GE. 1994. Natural enemies in straw-mulch reduce Colorado potato beetle populations and damage in potato. Biol Control 4:163-169.
Bulmer MG and JJ Bull. 1982. Models of polygenic sex determination and sex ratio evolution. Evolution 36:13-26.
Caprio MA. 1998. Evaluating resistance management strategies for multiple toxins in the presence of external refuges. J Econ Entomol 91:1021-1031.
Caprio M and E Grafius. 1990. Effects of light, temperature and feeding status on flight initiation in postdiapause Colorado potato beetle.Environ Entomol19:281-285.
Carrière Y and BE Tabashnik. 2001. Reversing insect adaptation to transgenic insecticidal plants. Proc R Soc Lond B 268:1475-1480.
Carriere Y, C Ellers-Kirk, R Biggs, B Degain, D Holley, C Yafuso, P Evans, TJ Dennehy and BE Tabashnik. 2005. Effects of cotton cultivar on fitness costs associated with of pink bollworm (Lepidoptera : Gelechiidae) to bt cotton. J Econ Entomol 98:947-954.
Casagrande RA. 1987. The Colorado potato beetle: 125 years of mismanagement. Bull Entomol Soc Am 33:142-150.
Clark JM 1997. Insecticides as tools in probing vital receptors and enzymes in excitable membranes. Pestic Biochem Physiol 57:235-254.
Clark JM, SH Lee, HJ Kim, KS Yoon and AG Zhang. 2001. DNA-based genotyping techniques for the detection of point mutations associated with insecticide resistance in Colorado potato beetle Leptinotarsa decemlineata. Pest Manage Sci 57:968-974.
Crow JF. 1957. Genetics of insect resistance. Annu Rev Entomol 2:227-246.
De Kort CAD. 1990. Thirty-five years of diapause research with the Colorado potato beetle. Entomol Exp Appl 56:1-13.
De Wilde J. 1948. Developpement embryonnaire et postembryonnaire dy doryphore (Leptinotarsa decemlineata Say) en function de la temperature. In: Proceedings 8th International Congress of Entomology, Stockholm, Sweden, pp. 310-321.
De Wilde J. 1962. The relation between diapause research and control of the Colorado potato beetle Leptinotarsa decemlineata Say. Ann Appl Biol 50:605-608.
Denholm I and MW Rowland. 1992. Tactics for managing pesticide resistance in arthropods: theory and practice. Annu Rev Entomol 37:91-112.
DiFonzo CD, DW Ragsdale, EB Radcliffe, NC Gunmestad and GA Secor. 1996. Crop borders reduce potato virus Y incidence in seed potato. Ann Appl Biol 129:289-302.
Dingle H. 1985. Migration. In: GA Kerkut and LI Gilbert (eds.), Insect physiology, biochemistry, and pharmacology, vol. 9. Pergamon Press, Oxford, England, pp. 375-415.
Dively GP, PA Follett, JJ Linduska and GK Roderick. 1998. Use of imidacloprid-treated row mixtures for Colorado potato beetle (Coleoptera: Chrysomelidae) management. J Econ Entomol 91:376-387.
Ferro DN. 1993. Potential for resistance to Bacillus thuringiensis: Colorado potato beetle (Coleoptera: Chrysomelidae) – a model system. Am Entomol 39:38-44.
Ferro DN. 1994. Biological control of the Colorado potato beetle. In: GW Zehnder, RK Jansson, ML Powelson, and KV Raman (eds), Advances in Potato Pest Biology and Management. APS Press, St. Paul, pp. 357-375.
Ferro DN, JA Logan, RH Voss and JS Elkinton. 1985. Colorado potato beetle (Coleoptera: Chrysomelidae) temperature-dependent growth and feeding rates. Environ Entomol 14:343-348.
Ferro DN, AF Tuttle and DC Weber. 1991. Ovipositional and flight behavior of overwintered Colorado potato beetle (Coleoptera: Chrysomelidae). Environ Entomol 20:1309-1314.
Ferro DN, AV Alyokhin and DB Tobin. 1999. Reproductive status and flight activity of the overwintered Colorado potato beetle.Entomol Exp Appl 91:443-448.
Feyereisen R. 2005. Insect Cytochrome P450. In: L Gilbert, K Latrou, and S Gill (eds). Comprehensive Molecular Insect Science. Elsevier, Amsterdam, Boston, pp 2-77.
Follett PA, GG Kennedy and F Gould. 1993. A simulation model that explores Colorado potato beetle (Coleoptera: Chrysomelidae) adaptation to insecticides. Environ Entomol 22: 283-296.
Follett PA, F Gould and GG Kennedy. 1995. High-realism model of Colorado potato beetle (Coleoptera: Chrysomelidae) adaptation to permethrin. Environ Entomol 24:167-178.
Forgash AJ. 1985. Insecticide resistance in the Colorado potato beetle. In: DN Ferro and RH Voss (eds), Proceedings of the Symposium on the Colorado Potato Beetle, 17th International Congress of Entomology. Massachusetts Experiment Station, University of Massachusetts, Amherst, MA, pp. 33-53.
French NM III, P Follett, BA Nault and GG Kennedy. 1993. Colonization of potato fields in eastern North Carolina by Colorado potato beetle. Entomol Exp Appl 68:247-256.
Gauthier NL, RN Hofmaster and M Semel. 1981. History of Colorado potato beetle control. In: JH Lashomb and R Casagrande (eds). Advances in Potato Pest Management. Hutchinson Ross Publishing Co., Stroudsburg, PA, pp. 13-33.
Gouamene-Lamine, CN, KS Yoon and JM Clark. 2003. Differential susceptibility to abamectin and two bioactive avermectin analogs in abamectin-resistant and -susceptible strains of Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera : Chrysomelidae). Pestic Biochem Physiol 76:15-23.
Grafius E. 1995. Is local selection followed by dispersal a mechanism for rapid development of multiple insecticide resistance in the Colorado potato beetle? Am Entomol 41:104-109.
Grafius E. 1997. Economic impact of insecticide resistance in the Colorado potato beetle (Coleoptera: Chrysomelidae) on the Michigan potato industry. J Econ Entomol 90:1144-1151.
Grafius EJ and DS Douches. 2008. The present and future role of insect-resistant genetically modified potato cultivars in potato IPM. In: J Romeis, AM Shelton, and GG Kennedy (eds.). Integration of Insect-Resistant GM crops within IPM Programs. Springer, New York (in press).
Harcourt DG. 1971. Population dynamics of Leptinotarsa decemlineata (Say) in eastern Ontario. III. Major population processes. Can Entomol 103:1049-1061.
Harris CR and HJ Svec. 1981. Colorado potato beetle resistance to carbofuran and several other insecticides in Quebec Leptinotarsa decemlineata. J Econ Entomol 74:421-424.
Harris CR and SA Turnbull. 1986. Contact toxicity of some pyrethroids insecticides, alone and in combination with piperonyl butoxide, to insecticide-susceptible and pyrethroid-resistant strains of the Colorado potato beetle (Coleoptera: Chrysomelidae) Can Entomol 118:1173-1176.
Hawthorne DJ. 2001. AFLP-based genetic linkage map of the Colorado potato beetle Leptinotarsa decemlineata: Sex chromosomes and a pyrethroid-resistance candidate gene. Genetics158:695-700.
Hawthorne DJ. 2003. Quantitative trait locus mapping of pyrethroid resistance in Colorado potato beetle, Leptinotarsa decemlineata (say) (Coleoptera: Chrysomelidae). J Econ Entomol 96:1021-1030.
Heim DC, GG Kennedy and JW Van Duyn. 1990. Survey of insecticide resistance among North Carolina Colorado potato beetle (Coleoptera: Chrysomelidae) populations. J Econ Entomol 83:1229-1235.
Heim DC, GG Kennedy, FL Gould and JW Van Duyn. 1992. Inheritance of fenvalerate and carbofuran resistance in Colorado beetles – Leptinotarsa decemlineata (Say) – from North Carolina. Pestic Sci 34:303-311.
Hitchner LS. 1952. The Insecticide Industry. In: Insects, the Yearbook of Agriculture 1952. House Doc. 413, Washington, DC, pp 450-457.
Hofmaster RN, RL Waterfield and JC Boyd. 1967. Insecticides applied to the soil for control of eight species of insects on Irish potatos in Virginia. J Econ Entomol 60:1311-1318.
Hough-Goldstein JA and JM Whalen. 1996. Relationship between crop rotation distance from previous potatoes and colonization and population density of Colorado potato beetle. J Agric Entomol 13:293-300.
Hough-Goldstein JA, GE Heimpel, HE Bechmann and CE Mason. 1993. Arthropod natural enemies of the Colorado potato beetle. Crop Protection 12:324-334.
Hoy CW and G Head. 1995. Correlation between behavioral and physiological responses to transgenic potatoes containing Bacillus thuringiensis delta-endotoxin in Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). J Econ Entomol 88:480-486.
Hoy CW, JA Wyman, TT Vaughn, DA East and P Kaufman. 1996. Food, ground cover, and Colorado potato beetle (Coleoptera: Chrysomelidae) dispersal in late summer. J Econ Entomol 89:963-969.
Hueth D and U Regev. 1974. Optimal agricultural pest management with increasing pest resistance. Am J Agric Econ 56:543-553.
Insecticide Resistance Action Committee (IRAC). 2008. IRAC mode of action classification, version 5.1. http://www.irac-online.org/documents/IRAC%20MoA%20Classification%20v5_3.pdf. Last accessed 15April, 2008.
Ioannidis PM, E Grafius and ME Whalon. 1991. Patterns of insecticide resistance to azinphosmethyl, carbofuran, and permethrin in the Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 84:1417-1423.
Ioannidis, PM, EJ Grafius, JM Wierenga, ME Whalon and RM Hollingworth. 1992. Selection, inheritance and characterization of carbofuran resistance in the Colorado potato beetle (Coleoptera, Chrysomelidae). Pestic Sci 35:215-222.
Isely D. 1935. Variations in the seasonal history of the Colorado potato beetle. J Kansas Entomol Soc 8:142-145.
Jacques RL. 1988. The Potato Beetles. E. J. Brill, Leiden.
Jermy T and G Saringer. 1955. A burgonyabogar (Leptinotarsa decemlineata Say). Mezögazd Kiadó, Budapest.
Johnson CG. 1969. Migration and Dispersal of Insects by Flight. Methuen and Co, London.
Jolivet P. 1991. The Colorado beetle menaces Asia (Leptinotarsa decemlineata Say 1824)(Col. Chrysomelidae). L’Entomologiste 47: 29-48.
Kearsey MJ and HS Pooni. 1996. The Genetical Analysis of Quantitative Traits. Chapman and Hall: London.
Kim HJ and JM Clark. 2002. Evaluation of resistant point mutations in recombinant acetylcholinesterase of Colorado potato beetle. Abstr Papers Am Chem Soc 224:U103-U103.
Kim HJ, DJ Hawthorne, T Peters, GP Dively and JM Clark. 2005. Application of DNA-based genotyping techniques for the detection of kdr-like pyrethroid resistance in field populations of Colorado beetle. Pestic Biochem Physiol 81:85-96.
Kim HJ, JB Dunn, KS Yoon and JM Clark. 2006. Target site insensitivity and mutational analysis of acetylcholinesterase from a carbofuran-resistant population of Colorado potato beetle, Leptinotarsa decemlineata (Say). Pestic Biochem Physiol 84:165-179.
Kim HJ, KS Yoon and JM Clark. 2007. Functional analysis of mutations in expressed acetylcholinesterase that result in azinphosmethyl and carbofuran resistance in Colorado potato beetle. Pestic Biochem Physiol 88:181-190.
Krishnan N, D Kodrik, F Turanli and F Sehnal. 2007. Stage-specific distribution of oxidative radicals and antioxidant enzymes in the midgut of Leptinotarsa decemlineata. J Insect Physiol 53:67-74.
Lactin DJ and Holliday NJ. 1994. Behavioral responses of Colorado potato beetle larvae to combinations of temperature and insolation, under field conditions. Entomol Exp Appl 72:255-263.
Lashomb JH and Y-S Ng. 1984. Colonization by the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Cerambycidae) in rotated and non-rotated potato fields. Environ Entomol 13:1352-1356.
Lee S and JM Clark. 1996. Tissue distribution and biochemical characterization of carboxylesterases associated with permethrin resistance in a near isogenic strain of Colorado potato beetle. Pestic Biochem Physiol 56:208-219.
Lee SH and JM Clark. 1998. Purification and characterization of multiple-charged forms of permethrin carboxylesterase(s) from the hemolymph of resistant Colorado potato beetle. Pestic Biochem Physiol 60:31-47.
Lee SH, JB Dunn, JM Clark and DM Soderlund. 1999. Molecular analysis of kdr-like resistance in a permethrin-resistant strain of Colorado potato beetle. Pestic Biochem Physiol 63:63-75.
Levine E, H Oloumi-Sadeghi and JR Fisher. 1992. Discovery of multiyear diapause in Illinois and South Dakota Northern corn rootworm (Coleoptera: Cerambycidae) eggs and incidence of the prolonged diapause trait in Illinois. J Econ Entomol 85:262-267.
Liu YB, BE Tabashnik, TJ Dennehy, AL Patin and AC Bartlett. 1999. Development time and resistance to Bt crops. Nature 400:519.
Liu ZW, MS Williamson, SJ Lansdell, ZJ Han, I Denholm and NS Millar. 2006. A nicotinic acetylcholine receptor mutation (Y151S) causes reduced agonist potency to a range of neonicotinoid insecticides. J Neurochem 99:1273-1281.
Logan PA, RA Casagrande, HH Faubert and FA Drummond. 1985. Temperature-dependent development and feeding of immature Colorado potato beetles, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Environ Entomol 14:275-283.
MacQuarrie CJK and G Boiteau. 2003. Effect of diet and feeding history on flight of Colorado potato beetle, Leptinotarsa decemlineata. Entomol Exp Appl 107:207-213.
May ML. 1981. Role of body temperature and thermoregulation in the biology of the Colorado potato beetle. In: JH Lashomb and R Casagrande (eds). Advances in Potato Pest Management. Hutchinson Ross Publishing Co., Stroudsburg, PA, pp 86-104.
McCauley DE and LM Reilly. 1984. Sperm storage and sperm precedence in the milkweed beetle, Tetraopes tetraophthalmus (Forster) (Coleoptera: Cerambycidae). Ann Entomol Soc Am 77:526-530.
McGaughey W and ME Whalon. 1992. Managing insect resistance to Bacillus thuringiensis toxins. Science 258:1451-1455.
McKenzie JA. 2000. The character or the variation: The genetic analysis of the insecticide-resistance phenotype. Bull Entomol Res 90:3-7.
Milner M, KJS Kung, JA Wyman, J Feldman and E Nordheim. 1992. Enhancing overwintering mortality of Colorado potato beetle (Coleoptera: Chrysomelidae) by manipulating the temperature of its diapause habitat. J Econ Entomol 85:1701- 1708.
Minder IF and DV Petrova. 1966. Ecological and physiological characteristics of the summer rest of the Colorado beetle. In: KV Arnoldi (ed.). Ecology and Physiology of Diapause in the Colorado Beetle. Nauka, Moscow, pp. 257–279.
Misener GC, G Boiteau and LP McMillan. 1993. A plastic-lining trenching device for the control of Colorado potato beetle: Beetle Excluder. Am Potato J 70:903- 908.
Miyo T, CBO Keil, JA Hough-Goldstein and Y Oguma. 1999. Inheritance of resistance to esfenvalerate in Colorado potato beetles (Coleoptera : Chrysomelidae). J Econ Entomol 92:1031-1038.
Mohammadi Sharif M, MJ Hejazi, A Mohammadi and MR Rashidi. 2007. Resistance status of the Colorado potato beetle, Leptinotarsa decemlineata, to endosulfan in East Azarbaijan and Ardabil provinces of Iran. 7pp. J Insect Sci 7:31, available online: insectscience.org/7.31.
Mota-Sanchez D 2003. Resistance and metabolism of imidacloprid in Colorado Potato Beetle, Leptinotarsa decemlineta Say (Coleoptera: Chrysomelidae), pp.122, Entomol. Michigan State University, East Lansing.
Mota-Sanchez D, RM Hollingworth, EJ Grafius and DD Moyer. 2006. Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera : Chrysomelidae). Pest Manage Sci 62:30-37.
Mowry TM and LE Sandvol. 1995. Survey of Colorado potato beetle insecticide resistance in Idaho. Am Potato J 72:551-558.
National Potato Council. 2008. Neonicotinoid insecticides: A grower approach to resistance management of Colorado potato beetle and green peach aphid in potato. http://www.nationalpotatocouncil.org/NPC/pressroom_newsflashes.cfm. Last accessed 7 April 2008).
Nauen R and I Denholm. 2005. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch Insect Biochem Physiol 58:200-215.
Ng Y-S and JH Lashomb. 1983. Orientation by the Colorado potato beetle (Leptinotarsa decemlineataSay). Anim Behav 31:617-618.
Noronha C, GM Duke, JM Chinn and MS Goettel. 2001. Differential susceptibility to insecticides by Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) populations from western Canada. Phytoprotection 82:113-121.
Olson ER, GP Dively and JO Nelson. 2000. Baseline susceptibility to imidacloprid and cross resistance patterns in Colorado potato beetle (Coleoptera: Chrysomelidae) populations. J Econ Entomol 93: 447-458.
Pourmirza AA. 2005. Local variation in susceptibility of Colorado potato beetle (Coleoptera: Chrysomelidae) to insecticide. J Econ Entomol 98:2176-2180.
Quinton RJ. 1955. DDT-resistant Colorado potato beetles? Proc North Centr Entomol Soc Am 9:94-95.
Rahardja U and M E Whalon. 1995. Inheritance of resistance to Bacillus thuringiensis subsp. tenebrionis CryIIIA delta-endotoxin in Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 88:21-26.
Rankin ME, ML McAnnely and JE Bodenhamer. 1986. The oogenesis-flight syndrome revisited. In: W Danthanarayana (ed), Insect flight, dispersal, and migration. Springer-Verlag, New York, pp. 27-48.
Roderick GK, LG De Mendoza, GP Dively and PA Follett. 2003. Sperm precedence in Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae): Temporal variation assessed by neutral markers. Ann Entomol Soc Am 96:631-636.
Rose RL and WA Brindley. 1985. An evaluation of the role of oxidative enzymes in Colorado potato beetle resistance to carbamate insecticides. Pestic Biochem Physiol 23:74-84.
Silcox CA, GM Ghidiu and A.J. Forgash. 1985. Laboratory and field evaluation of piperonyl butoxide as a pyrethroid synergist against the Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 78:1399-1405.
Soderlund DM, CW Hessney and MG Jiang. 1983. Metabolism of fenvalerate by resistant Colorado potato beetles adults. Abstr Papers Am Chem Soc 186:28-PEST.
Solbreck C. 1978. Migration, diapause, and direct development as alternative life histories in a seed bug, Neacoryphus bicruci. In: H Dingle (ed), Evolution of Insect Migration and Diapause. Springer, New York, pp.195-217.
Stankovic S, A Zabel, M Kostic, B Manojlovic and S Rajkovic. 2004. Colorado potato beetle [Leptinotarsa decemlineata (Say)] resistance to organophosphates and carbamates in Serbia. J Pest Sci 77:11-15.
Stegwee D, EC Kimmel, JA de Boer and S Henstra. 1963. Hormonal control of reversible degeneration of flight muscle in the Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera). J Cell Biol 19:519-527.
Stone BF. 1968. A formula for determining degree of dominance in cases of monofactorial inheritance of resistance to chemicals. Bull WHO 38:325-326.
Stoner KA. 1993. Effects of straw and leaf mulches and sprinkle irrigation on the abundance of Colorado potato beetle (Coleoptera: Chrysomelidae) on potato in Connecticut. J Entomol Sci 28:393-403.
Stewart JG, GG Kennedy and AV Sturz. 1997. Incidence of insecticide resistance in populations of Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), on Prince Edward Island. Can Entomol 129:21- 26.
Szentesi A. 1985. Behavioral aspects of female guarding and inter-male conflict in the Colorado potato beetle. In: DN Ferro and RH Voss (eds), Proceedings of the Symposium on the Colorado Potato Beetle, 17th International Congress of Entomology. Massachusetts Experiment Station, University of Massachusetts, Amherst, MA, pp. 127-137.
Tabashnik BE. 1994. Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol 39:47-79.
Tan JG, D Mota-Sanchez, VL Salgado and RM Hollingworth. 2005. Decreased nicotinic sensitivity to imidacloprid as a resistance mechanism in the Colorado potato beetle, Leptinotarsa decemlineata (SAY). Abstr Papers Am Chem Soc 229:U68-U68.
Tan JG, V Salgado and RM Hollingworth. 2008. Neural actions of imidacloprid and their involvement in resistance in the Colorado potato beetle, Leptinotarsa decemlineata (Say). Pest Manage Sci 64:37-47.
Tauber MJ and CA Tauber. 2002. Prolonged dormancy in Leptinotarsa decemlineata (Coleoptera: Chrysomelidae): a ten-year field study with implications for crop rotation. Environ Entomol 31:499-504.
Trisyono A and ME Whalon. 1997. Fitness costs of resistance to Bacillus thuringiensis in Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 90:267-271.
Trouvelot B. 1936. Remarques sur l’ecologie du doryphore en 1935 dans le massif central et le centre de la France. Rev Zool Agr Appl 25: 33-37.
Ushatinskaya RS. 1962. Colorado potato beetle diapause and development of its multi-year infestations. Zashchita Rastenii 6:53-54.
Ushatinskaya RS. 1966. Prolonged diapause in the Colorado beetle and conditions of its development. In: KV Arnoldi (ed.). Ecology and Physiology of Diapause in the Colorado Beetle. Nauka, Moscow, pp 120–143.
Vlasova VA. 1978. A prediction of the distribution of Colorado beetle in the Asiatic territory of the USSR. Zashchita Rastenii 6:44-45.
Voss RH and DN Ferro. 1990. Phenology of flight and walking by Colorado potato beetle (Coleoptera: Chrysomelidae) adults in western Massachusetts. Environ Entomol 19:117-122.
Walgenbach JF and JA Wyman. 1984. Colorado potato beetle (Coleoptera: Chrysomelidae) development in relation to temperature in Wisconsin. Ann Entomol Soc Am 77:604-609.
Weber DC and DN Ferro. 1993. Distribution of overwintering Colorado potato beetle in and near Massachusetts potato fields. Entomol Exp Appl 66:191-196.
Weber DC and DN Ferro. 1994. Colorado potato beetle: diverse life history poses challenge to management. In: GW Zehnder, RK Jansson, ML Powelson, and KV Raman (eds), Advances in Potato Pest Biology and Management. APS Press, St. Paul, pp 54-70.
Weber DC and DN Ferro. 1996. Flight and fecundity of Colorado potato beetles fed on different diets. Ann Entomol Soc Am 89:297-306.
Weber D. 2003. Colorado beetle: pest on the move. Pestic Outlook 14:256-259.
Wegorek W. 1957a. Badania nad biologia i ekologia stonki ziemniaczanej (Leptinotarsa decemlineata Say). Roczn Nauk Roln 74A2: 135-185.
Wegorek W. 1957b. Badania nad zimovaniem stonki ziemniaczanej (Leptinotarsa decemlineata Say). Roczn Nauk Roln 74A2: 316-338.
Weisz R, Z Smilowitz and B Christ. 1994. Distance, rotation, and border crops affect Colorado potato beetle (Coleoptera: Chrysomelidae) colonization and population density and early blight (Alternaria solani) severity in rotated potato fields. J Econ Entomol 87:723-729.
Weisz R, Z Smilowitz and S Fleischer. 1996. Evaluating risk of Colorado potato beetle (Coleoptera: Chrysomelidae) infestation as a function of migratory distance. J Econ Entomol 89:435-441.
Whalon ME and DN Ferro. 1998. Bt-potato resistance management. In: M Mellon and J Rissler (eds), Now or never: serious new plans to save a natural pest control. UCS, Cambridge, MA, pp. 107-136.
Whalon ME, DL Miller, RM Hollingsworth, EJ Grafius and JR Miller. 1993. Selection of a Colorado potato beetle (Coleoptera: Chrysomelidae) strain resistant to Bacillus thuringiensis. J Econ Entomol 86:226-233.
Whalon ME, D Mota-Sanchez and RM Hollingworth. 2008. The MSU Arthropod pesticide resistance database. Available online: http://www.pesticideresistance.org. Last accessed 7 April, 2008.
Wierenga JM and RM Hollingworth. 1992. Inhibition of insect acetylcholinesterase by the potato glycoalkaloid -chaconine. Natur Tox 1:96-99.
Wierenga JM and RM Hollingworth. 1993. Inhibition of altered acetylcholinesterases from insecticide-resistant Colorado potato beetles (Coleoptera: Chrysomelidae). J Econ Entomol 86:673-679.
Wierenga JM and RM Hollingworth. 1994. The role of metabolic enzymes in insecticide-resistant Colorado potato beetles. Pestic Sci 40:259-264.
Wierenga JM, DL Norris and ME Whalon. 1996. Stage-specific mortality of Colorado potato beetle (Coleoptera: Chrysomelidae) feeding on transgenic potatoes. J Econ Entomol 89:1047-1052.
Wiktelius S. 1981. Wind dispersal of insects. Grana 20:205-207.
Worner SP. 1988. Ecoclimactic assessment of potential establishment of exotic pests. J Econ Entomol 81:973-983.
Wright RJ. 1984. Evaluation of crop rotation for control of Colorado potato beetle (Coleoptera: Chrysomelidae) in commercial potato fields on Long Island. J Econ Entomol 77:1254-1259.
Yang B. 1994. Muscle development, energy source utilization, and metabolism hormone activity in Colorado potato beetle, Leptinotarsa decemlineata (Say) flight. M.S. Thesis, University of Massachusetts.
Yoon KS, JO Nelson and JM Clark. 2002. Selective induction of abamectin metabolism by dexamethasone, 3-methylcholanthrene, and phenobarbital in Colorado potato beetle, Leptinotarsa decemlineata (Say). Pestic Biochem Physiol 73:74-86.
Zehnder GW. 1986. Timing of insecticides for control of Colorado potato beetle (Coleoptera: Chrysomelidae) in eastern Virginia based on differential susceptibility of life stages. J Econ Entomol 79:851-856.
Zehnder GW and WD Gelernter. 1989. Activity of the M-ONE formulation of a new strain of Bacillus thuringiensis against the Colorado potato beetle (Coleoptera: Chrysomelidae): relationship between susceptibility and insect life stage. J Econ Entomol 82:756-61.
Zehnder GW and J Hough Goldstein. 1990. Colorado potato beetle (Coleoptera: Chrysomelidae) population development and effects on yield of potatoes with and without straw mulch. J Econ Entomol 83:1982-1987.
Zhao JZ, BA Bishop and EJ Grafius. 2000. Inheritance and synergism of resistance to imidacloprid in the potato beetle (Coleoptera : Chrysomelidae). J Econ Entomol 93:1508-1514.
Zhu KY and JM Clark. 1995. Cloning and sequencing of a cDNA encoding acetylcholinesterase in Colorado potato beetle, Leptinotarsa decemlineata (Say). Insect Biochem Mol Biol 25:1129–1138.
Zhu KY and JM Clark. 1997. Validation of a point mutation of acetylcholinesterase in Colorado potato beetle by polymerase chain reaction coupled to enzyme assay. Pestic Biochem Physiol 57:28-35.
Zhu KY, SH Lee and JM Clark. 1996. A point mutation of acetylcholinesterase associated with azinphosmethyl resistance and reduced fitness in Colorado potato beetle. Pestic Biochem Physiol 55:100-108.
